By Lynn McCain
Idiopathic Pulmonary Fibrosis (IPF) is a disease with unknown causes that result in scarring of the lungs. The scar tissue forms on the alveoli, the small air sacs in the lungs, which makes it more difficult for oxygen to pass through the alveoli walls and into the bloodstream and for carbon dioxide to be removed. The scarring is permanent and the disease is unpredictable – no one knows who will get it or how quickly it will progress, but it always progresses. As it progresses, lung function decreases making it harder to breathe. Patients go from feeling winded to requiring oxygen supplementation, and in advanced disease, patients may die. Currently, there is no cure for IPF although a couple of drugs are now available to slow down disease progression in certain patients.
A recently published research study led by Drs. Sem Phan and Tianju Liu from the Department of Pathology reported new findings that could help scientists predict disease progression and suggest a new immunotherapy target for the treatment of IPF and other fibrotic lung diseases.
The immune system is made up of multiple types of white blood cells, one of which is called a T-cell. Prior research found that a protein called B7-H3 (CD276) plays a role in activating T-cells to fight off disease, but recent discoveries suggest a more significant role in suppressing or inhibiting T-cell activation. Furthermore, when the levels of this protein increase, cancer patients worsen. “Cancer stem cells utilize B7-H3 to evade immune surveillance for cancer initiation and metastasis,” explained Liu. “Interestingly, carcinoma-associated fibroblast (CAF) derived B7-H3 promotes an immunosuppressive microenvironment by recruiting Tregs, thus acquiring resistance to immunotherapies.” Scientists are now working to find ways to target Tregs and other cells that inhibit the immune system as potential immunotherapy against cancer.
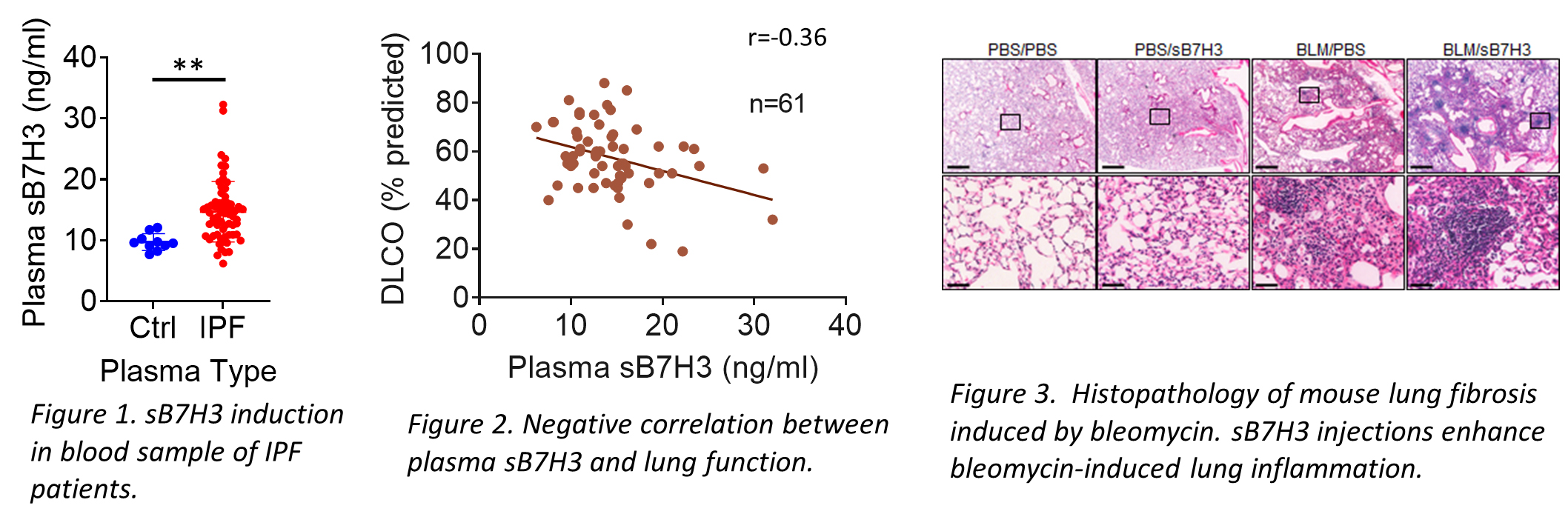
In addition to its importance in cancer, Phan and Liu’s research team discovered that B7-H3 is also elevated in IPF patients’ blood as well as its soluble cleavage product (sB7-H3) in IPF patients’ plasma. They found that sB7-H3 levels are significantly correlated with a decline in lung function. “Treating mice with sB7-H3 protein results in an increased number of inflammatory cells, elevated level of inflammatory gene expression, and expansion of myeloid-derived suppressor cells (MDSCs) in both bone marrow and lung” reported Phan. This suggests bone marrow-derived MDSCs may be recruited to the lung to promote and/or exacerbate fibrosis in IPF patients.
These findings uncovered a new role for the immune checkpoint marker (B7-H3) in lung fibrosis that can potentially serve as a novel target for immunotherapy to slow down or abort the progression of lung fibrosis in patients with IPF and other chronic lung fibrotic diseases. In addition, sB7H3 levels in the plasma could serve as a potential marker to predict how quickly the disease progresses in patients and assess responsiveness to therapy, allowing for more informed treatment decisions.
—
To read more about this study:
B7H3 expression and significance in idiopathic pulmonary fibrosis.
Chuling Fang, Andrew E Rinke, Jing Wang, Kevin R Flaherty, Sem H. Phan and Tianju Liu.
Journal of Pathology. 2022; 256: 310–320. DOI: 10.1002/path.5838